Activation Energy on a Graph: The Hidden Catalyst Shaping Reaction Speeds
Activation Energy on a Graph: The Hidden Catalyst Shaping Reaction Speeds
Visually depicting the energy barriers that govern chemical reactions—activation energy—on a graph reveals far more than a plot of numbers alone. It tells the story of why some reactions proceed rapidly, while others linger unyielding, locked behind invisible walls of energy. The activation energy graph stands as a cornerstone of physical chemistry, offering scientists precise insights into reaction dynamics, mechanisms, and control.
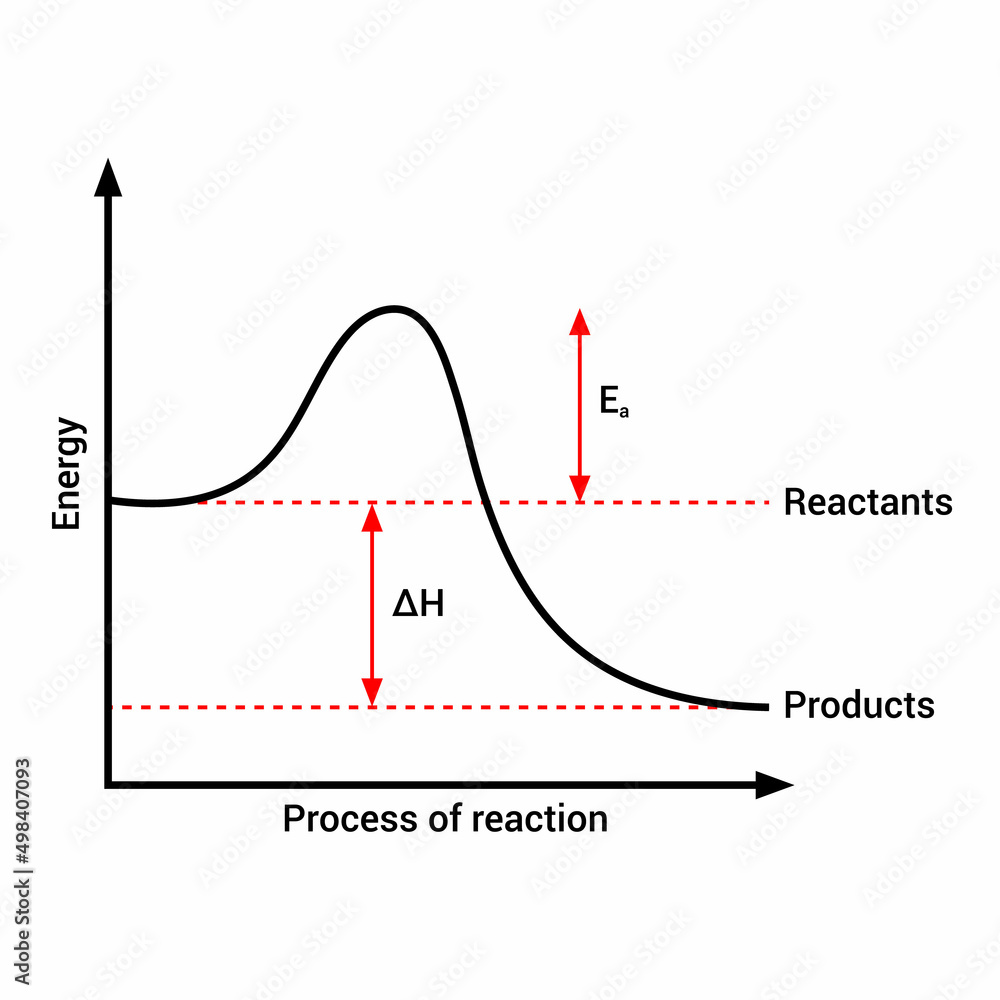
By interpreting the vertical threshold separating reactants from products, researchers decode how temperature, catalysts, and molecular structure influence reaction rates—insights critical to fields ranging from industrial synthesis to pharmaceutical development. Activation energy, measured in kilojoules per mole (kJ/mol), represents the minimum energy required for a chemical transformation to occur. On a standard Arrhenius plot—a graph of reaction rate versus inverse temperature—the activation energy appears as the vertical intercept’s energy gap relative to the fraction of molecules overcoming the barrier.
This departure from a simple energy landscape underscores the kinetic bottleneck that dictates reaction feasibility. As described by chemist John A. STSEVENJEN—whose pioneering work clarified kinetic energy distributions—“The activation energy is the chasm between chaos and coordination: a small hurdle enables speed, while a steep wall halts progress.”
Understanding this concept purely through data obscures its real-world potency.
Consider the Arrhenius equation: k = A·e⁻鲡Eₐ⁄RT where k is the rate constant, A is the frequency factor, Eₐ is activation energy, R is the gas constant, and T is temperature. The exponential term e⁻ᵀᴇⁱᵈ(Eₐ/R) governed by activation energy reveals its outsized influence. Even minor reductions in Eₐ—such as those afforded by catalysts—can exponentially accelerate reactions, explaining why enzymes in living cells achieve astonishing efficiency.
In essence, activation energy is not just a parameter—it is the key lever controlling chemical time itself.
Activation energy graphs transform abstract molecular behavior into visual, actionable data. On such a graph, the y-axis typically records the reaction’s energy, while the x-axis plots temperature or, alternatively, inverse rate, with the activation energy manifesting as a vertical offset between reactant and transition state energies. A steeper rise from reactant to transition state signals higher kinetic resistance, often linked to complex reaction mechanisms involving multiple steps or unstable intermediates.
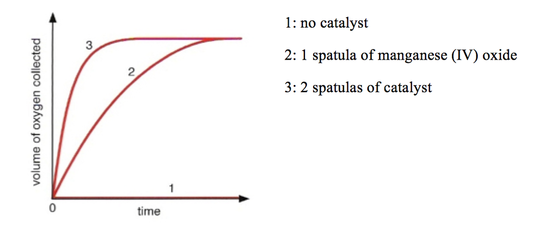
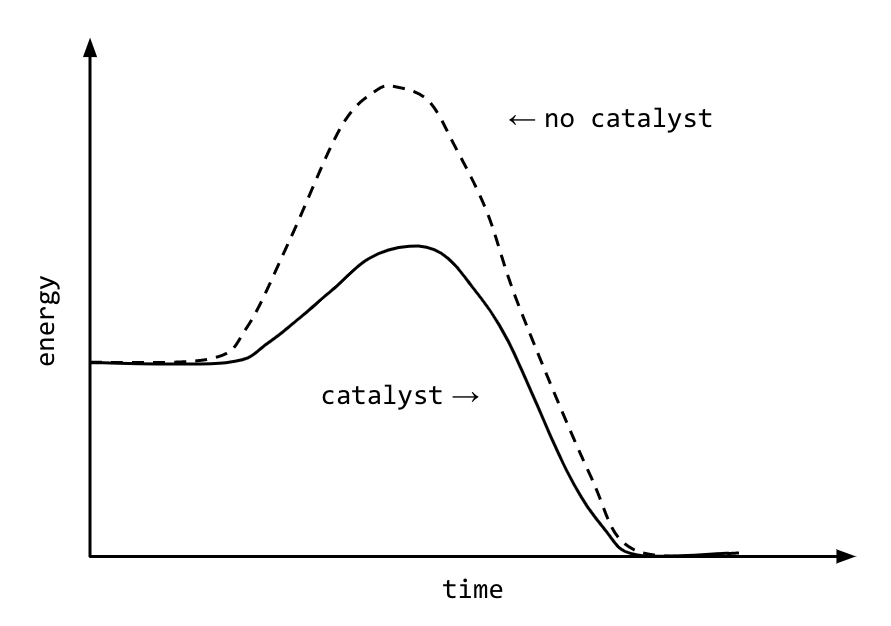
Take the decomposition of hydrogen peroxide (H₂O₂), a classic demonstration often used in education: at room temperature, H₂O₂ persists as a stable compound due to its high activation energy. But when catalyzed by manganese dioxide, the graph shows a dramatic drop in Eₐ, making the breakdown proceed rapidly within seconds. “The graph proves what experiments confirm: catalysts don’t create new energy,” explains physical chemist Dr.
Elena Marquez. “They lower the energy barrier, effectively shrinking the obstacle so molecules succeed by sheer statistical probability.” This principle extends beyond academic labs—industrial processes rely on manipulating activation energy to optimize yield and efficiency, from petrochemical refining to anhydrous synthesis of key pharmaceuticals.
Understanding activation energy through graphical analysis also illuminates reaction pathways.
Reaction coordinate diagrams, which accompany activation energy plots, reveal not only Eₐ but also the stability of intermediates and the feasibility of alternative routes. In mechanism studies, deviations from a single-step rate prediction often expose multi-step processes, each with its own energy hurdle. Advanced computational chemistry now integrates activation energy data to simulate reaction trajectories, guiding synthetic design with unprecedented precision.
Moreover, activation energy graphs vary by condition. Increasing temperature shifts the rate curve upward in slope, effectively reducing the relative energy gap molecules must bridge—though without lowering Eₐ itself. This explains why warmth fuels biological metabolism and industrial heating, but also why uncontrolled temperature swings compromise chemical stability.
The graph thus becomes a diagnostic tool, revealing both vulnerabilities and leverage points in reaction design.
Examples abound where mastering activation energy has driven innovation. The Haber-Bosch process, pivotal for global ammonia production, hinges on iron-based catalysts reducing Eₐ for nitrogen-hydrogen bond breaking.
Similarly, catalytic converters in automobiles use platinum-group metals to lower activation barriers for harmful exhaust oxidation, transforming toxic gases into less damaging compounds at ambient temperatures. These successes underscore one key truth: activation energy is not a barrier to be brute-forced, but a variable to be intelligently managed.
As research advances, activation energy graphs continue to evolve in complexity and utility.
Modern techniques like transition state theory and molecular dynamics simulations feed high-resolution energy landscapes into predictive models, accelerating discovery in fields from materials science to green chemistry. The graph, once a simple tool for quantifying energy, now serves as a dynamic interface between theory and application—revealing how a mere increase or decrease in energy can reshape the fate of molecules, reactions, and entire industrial processes.
Activation energy on a graph is far more than a line between numbers—it is a narrative of molecular struggle and triumph, a visual chronicle of kinetics in action.
Its clarity empowers scientists to predict, manipulate, and optimize reactions with precision unmatched by trial and error. In a world where chemical efficiency drives progress, understanding this energy threshold remains indispensable. The graph does not just chart energy—it charts possibility.

Related Post

Demystifying IEWS Huawei: What This Sophisticated Network Technology Truly Means

Why Grey’s Anatomy Bosses Wrote Off Cristina Yang: A Shift in Narrative Priorities

Saudi Arabia’s Kings: The Royal Family’s Enduring Legacy of Power, Vision, and Transformation

Hi Five Vs High Five: The Debate That’s Ranging Through Social Media and Sports Culture

