Decoding Calcium’s Symbol: Lewis Symbol of Ca Reveals Key to Its Chemical Identity
Decoding Calcium’s Symbol: Lewis Symbol of Ca Reveals Key to Its Chemical Identity
The silent force behind human bone formation and essential in biological signaling, calcium (Ca) remains a cornerstone of chemistry and life alike. At the heart of understanding this vital element lies its Lewis symbol — a deceptively simple representation that captures the atom’s proton count, electron configuration, and bonding behavior. Examining Lewis Symbol of Ca offers more than a visual convenience — it reveals fundamental insights into calcium’s role across biology, industry, and geology.
Beyond a mere chemical shorthand, this symbol serves as a bridge between abstract electron theory and tangible real-world applications, from bone health to industrial catalysts.
The Lewis Symbol Explained: Unpacking Calcium’s Atomic Blueprint
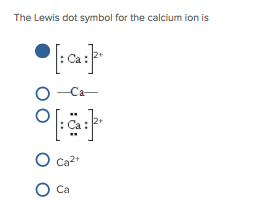
Calcium is represented by the Lewis symbol Ca: Ca· This concise symbol encodes critical data: one uppercase “Ca” denotes the atomic number — the number of protons in its nucleus — while the central dot signifies the six valence electrons occupying the outermost shell. These valence electrons define calcium’s chemical reactivity and predominant oxidation state.The symbol follows strict conventions of the Lewis notation, emphasizing clarity and precision. Despite its simplicity, Ca’s Lewis symbol unlocks a layered understanding: - **Atomic Number**: 20 — a key identifier shared with other alkaline earth metals. - **Electron Configuration (Valence)**: 1s² 2s² 2p⁶ 3s² (or fully: 1s² 2s² 2p⁶ 3s²), emphasizing the two electrons in the outermost shell.
- **Common Oxidation State**: +2, arising from the loss of the two 4s valence electrons. This visual shorthand allows scientists and learners alike to instantly grasp calcium’s electron architecture without delving into orbital formalism.
The Significance of Calcium’s Electron Structure
The distribution of valence electrons directly influences calcium’s chemical behavior.With a full outer shell of six electrons, calcium sits at the edge of stability — eager to shed electrons and form +2 cations. This tendency drives its reactivity, especially with nonmetals like oxygen and halogens. The Lewis symbol’s depiction of six valence electrons illuminates why calcium readily forms ionic bonds.
When combined with negatively charged anions, calcium transmits electrons completely, yielding compounds such as calcium oxide (CaO) and calcium chloride (CaCl₂). These transformations underpin critical applications, from cement production to water treatment. Supporting this behavior, calcium’s 3s² electron shell provides structural stability yet allows facile electron loss.
“The ease with which calcium donates two electrons reflects its position in Group 2 of the periodic table — a classical example of the ‘duo electron’ ion,” notes Dr. Elena Marquez, a periodic table specialist at the International Union of Pure and Applied Chemistry.
From Biology to Industry: Calcium’s Lewis Symbol in Action
In human physiology, calcium’s Lewis symbol directly correlates with its indispensable biological roles.The ion Ca²⁺ regulates nerve transmission, muscle contraction, and blood clotting — processes all reliant on the ion’s stable, positively charged structure. Calcium ions bind to proteins like calmodulin, triggering cellular responses that shape everything from bone density to heart rhythm. “The simplicity of the Ca²⁺ ion’s charge allows precise control in biological systems,” explains Dr.
James Tran, a biochemist at Stanford Medical Center. “Calcium’s moderate reactivity ensures it remains regulated, avoiding unchecked activity that could damage cells.” Beyond biology, calcium’s symbolic clarity facilitates its industrial handling. In cement manufacturing, CaO reacts with water to form calcium hydroxide, a key component in Portland cement.
In electronics, calcium compounds feature in phosphors and sensors. The Lewis symbol enables engineers and chemists to anticipate these behaviors without unnecessary complexity. Industrial chemists often rely on Lewis notation to model reactions involving calcium.
For instance, the reaction: Ca + 2HCl → CaCl₂ + H₂↑ is instantly parseable: calcium donates electrons to transfer hydrogen, producing calcium chloride and hydrogen gas. Each role — metal, ion, reactant — visually communicated in seconds.
The calcium ion’s Lewis symbol — particularly the +2 charge — enables it to bind selectively to receptor sites on cell surfaces and intracellular proteins. This binding triggers conformational shifts that initiate signaling cascades. One striking example is in neuromuscular junctions, where calcium influx into nerve terminals causes synaptic vesicles to fuse with membranes and release neurotransmitters.
“The rapid on-off switching of calcium concentration is governed by its ionic form’s ability to intercalate and displace,” Tran observes. “Calcium’s +2 charge ensures strong but reversible interaction with charge-sensitive proteins like troponin and calmodulin.” Moreover, calcium’s role in bone mineralization hinges on its ionic state. Hydroxylapatite crystals — the primary mineral in bone — depend on Ca²⁺ ions bonding with phosphate.
“Without the validated presence of calcium ions, bone structure would collapse,” Marquez observes. “The Lewis symbol makes this connection obvious: a single calcium ion can anchor an entire crystalline framework.”
Calcium oxide absorbs carbon dioxide in industrial flue gases, a reaction predictable from calcium’s tendency to form stable carbonates. Carbon capture: CaO + CO₂ → CaCO₃ This endothermic reaction relies on calcium’s standard +2 charge and oxides’ reactivity — both clear from its symbol. Energy storage also benefits.
Calcium ion batteries, though still developing, leverage the reversible +2 charge to shuttle between electrodes, offering potential for safer, higher-capacity storage than lithium alternatives. In consumer products, calcium’s role extends to food fortification. Calcium-fortified plant milks and cereals depend on biochemically stable calcium salts — forms directly describable via Ca²⁺.
“The reproducibility of the Lewis symbol allows consistent formulation every time,” notes food chemist Dr. Leila Okafor. “It ensures predictable dissolution and bioavailability.”
Environmental technologies similarly benefit.
Calcium-based builders in wastewater treatment precipitate heavy metals, while lime-stabilized soils use calcium hydroxide to neutralize acidity. Each application roots itself in calcium’s ionic form — a truth elegantly summarized by its symbol.
As research expands into sustainable materials and medical therapeutics, the symbol remains a constant pointer to calcium’s central role. In a world increasingly shaped by precision chemistry, the Ca symbol endures not just as a chemical shorthand, but as a beacon of clarity. It connects the abstract quantum world — electrons and orbitals — with tangible outcomes: healthy bones, clean air, and innovative technologies.
For scientists, educators, and innovators alike, understanding the Lewis Symbol of Ca is more than academic — it is the first step toward harnessing calcium’s full potential.

Related Post
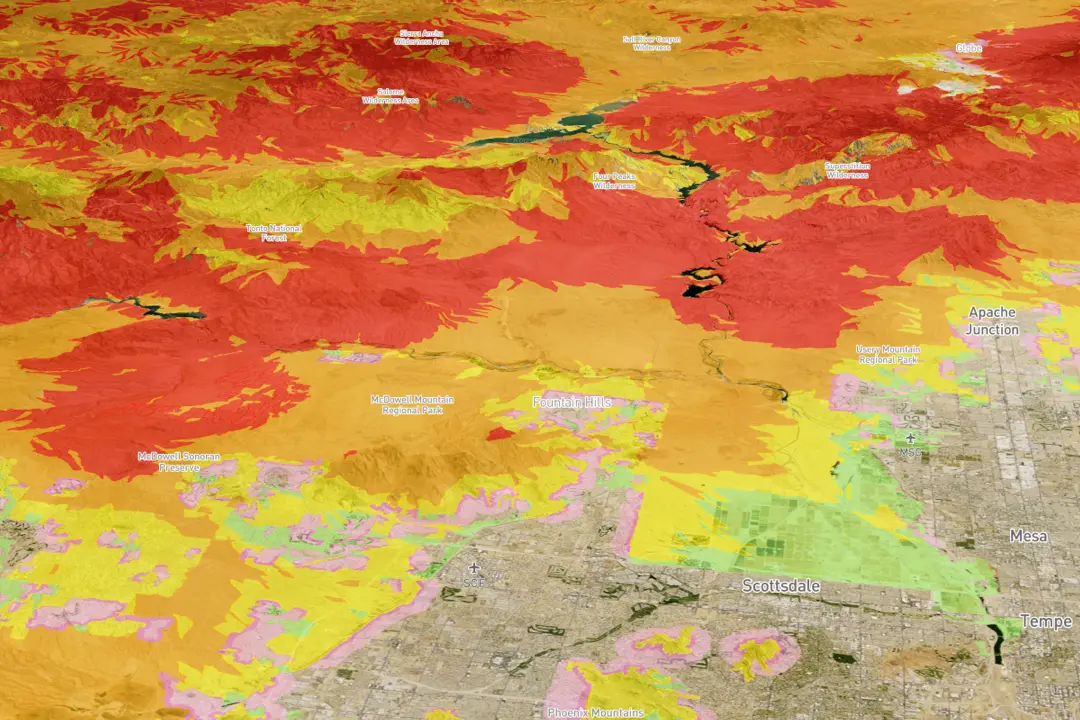
California’s Current Season What to Expect: Spring Sizzles with Wildfire Risk, Bountiful Harvest, and Cultural Revival
Who is Quinlin Dempsey Stiller Everything About Ben Stillers son
Alan Jackson Hospitalized: The Historic Singer’s Battle, Medical Timeline, and Hopeful Recovery Journey
Delving into the Enduring Inheritance of Powerhouse Michelle Rodriguez Through Her Defiant Parts

