Mastering Fluid Balance: The Critical Role of Isotonic, Hypotonic, and Hypertonic Solutions in Clinical Care
Mastering Fluid Balance: The Critical Role of Isotonic, Hypotonic, and Hypertonic Solutions in Clinical Care
In the intricate landscape of human physiology, precise fluid dynamics determine health outcomes—whether in emergency rooms, operating the rooms, or intensive care units. At the heart of this balance lie three pivotal concepts: isotonic, hypotonic, and hypertonic solutions, each playing a distinct role in maintaining cellular equilibrium and optimizing patient care. Understanding how these solutions—defined by their solute concentration gradients—interact with bodily fluids reveals transformative principles in medical hydration, infusion therapy, and treating fluid disorders.
These solutions are not abstract biochemical terms; they are essential tools clinicians rely on daily to stabilize patients, correct electrolyte imbalances, and support vital organ function.
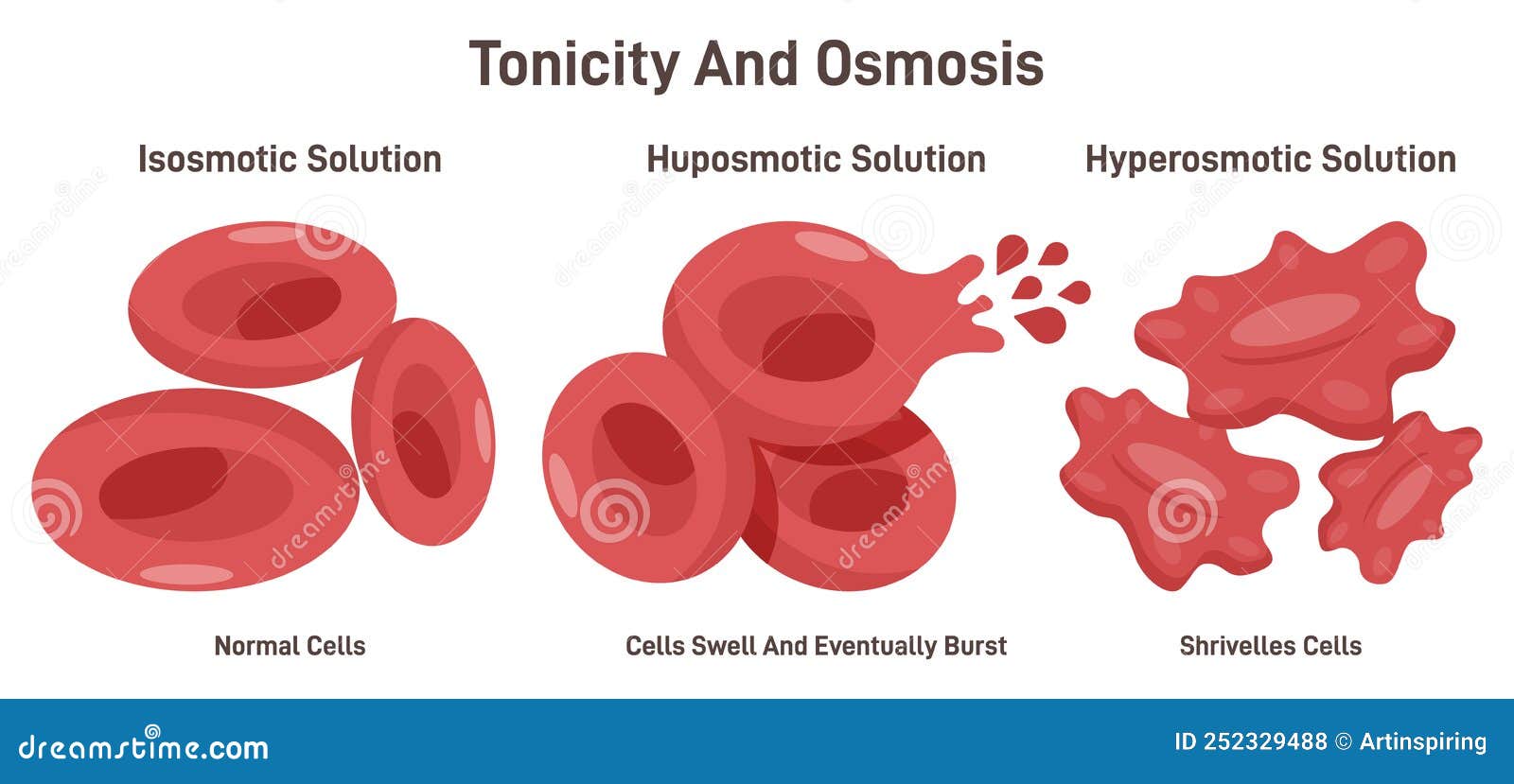
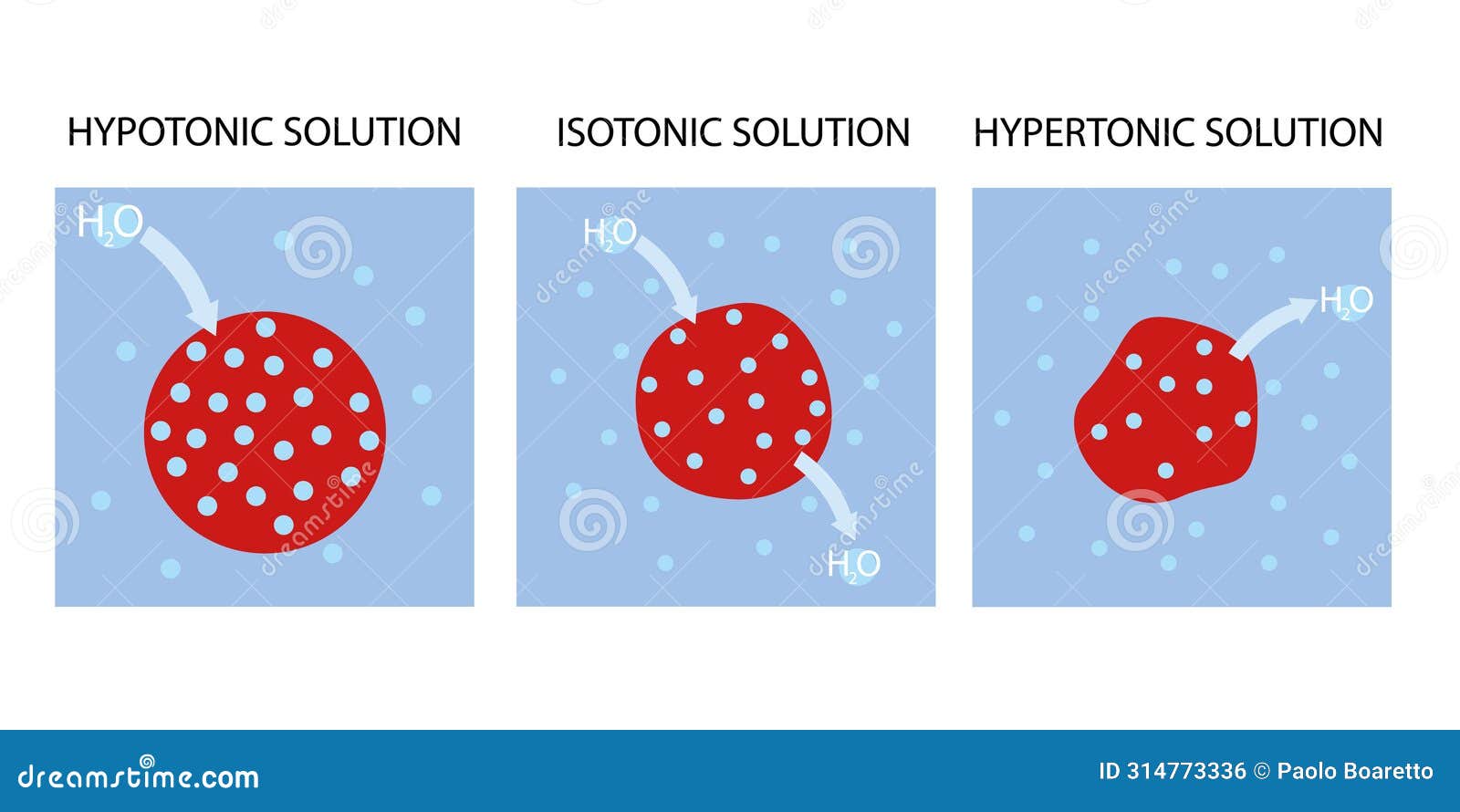
At the core, fluid solution classification hinges on osmolarity—the concentration of dissolved particles in a solution relative to bodily fluids. Isotonic solutions, with an osmolarity roughly equal to that of human blood (approximately 290 mOsm/L), enable water and solutes to flow freely across cell membranes without net movement.
This makes them ideal for general volume replacement, ensuring cells neither swell nor shrink. In contrast, hypotonic solutions—having lower osmolarity than blood—drive water into cells, expanding plasma volume gently. Hypertonic solutions, conversely, possess higher solute concentration, pulling fluid out of cells toward the interstitial space.
This powerful shift empowers rapid correction of severe dehydration or intracranial pressure, but demands careful clinical oversight. Each solution type serves a deliberate purpose, and their appropriate use reflects both scientific understanding and clinical acumen.
Isotonic Solutions: The Gold Standard for Volume Expansion
Isotonic solutions function as molecular neutralizers, maintaining equilibrium between intravascular and interstitial compartments. With an osmolarity matching human plasma, these solutions cause no net movement of fluid into or out of cells, making them the safest option for most volume replacement scenarios.Sodium chloride 0.9% (normal saline) and Ringer’s lactate are prime examples, widely used in trauma resuscitation, surgical fluid administration, and intravenous hydration.
The benefits of isotonic fluids extend beyond simple volume support. By preserving cellular integrity, they reduce risks of edema or hemolysis—complications densely associated with inappropriate infusion choice.
For instance, in postoperative patients experiencing hypovolemia, an isotonic solution rapidly restores perfusion pressure without compromising cell morphology. According to clinical guidelines, isotonic solutions are preferable for initial resuscitation in shock states unless specific indications demand otherwise. Moreover, in patients with chronic kidney disease or electrolyte imbalances, isotonic fluids minimize osmotic stress, promoting hemodynamic stability.
Clinical Applications: When to Trust Isotonic Solutions
- **Trauma resuscitation**: Rapid restoration of intravascular volume prevents organ hypoperfusion.- **Postoperative hydration**: Prevents hypovolemic shock after major surgery. - **General fluid replacement**: Municipal water and IV normal saline are staples in outpatient and hospital settings. - **Compensation for insensible losses**: Burns, fever, and excessive vomiting deplete intravascular volume; isotonic solutions offset this efficiently.
While highly effective, isotonicity is not universally optimal—its neutral effects require context. In patients with cellular edema or acute intracranial hypertension, alternative approaches take precedence. Nonetheless, isotonic solutions remain foundational, offering a reliable bridge until more targeted therapies are implemented.
Hypotonic Solutions: The Gentle Expanders of Plasma Volume
Hypotonic solutions, characterized by lower solute concentration than blood, initiate a controlled influx of fluid into cells and interstitial spaces. This osmotic gradient draws water inward, expanding extracellular volume while minimizing cellular swelling—critical in conditions requiring careful hydration management. Sodium phosphate and hypertonic saline diluted to <0.9% or formulations like 5% dextrose in water (when uncompensated) exemplify hybrid or supervised hypotonic infusions.Despite their therapeutic potential, hypotonic solutions demand precision. Their use in patients with impaired renal function or already elevated intracellular fluid risks cellular swelling, particularly in the brain. When used appropriately, however, they excel in specific clinical niches.
Diluted dextrose with insulin (D5+insulin) in hyponatremia corrects serum sodium levels without triggering osmotic demyelination—an example of targeted hypotonic intervention. Similarly, minimal hypotonic fluids may support renal perfusion in select hypovolemic states, provided solute shifts remain balanced.


Related Post

Iran SS Anime Strike: Unraveling the Cultural Phenomenon Shaping a New Generation
Charlotte Flair Replaced On SmackDown Banner After Recent Injury

Unlocking Roblox Photo Ids: The Visual Language That Defines Your Digital Identity
Joey Gallo Tourettes and Facial Tics What happened to the Twitch star face

