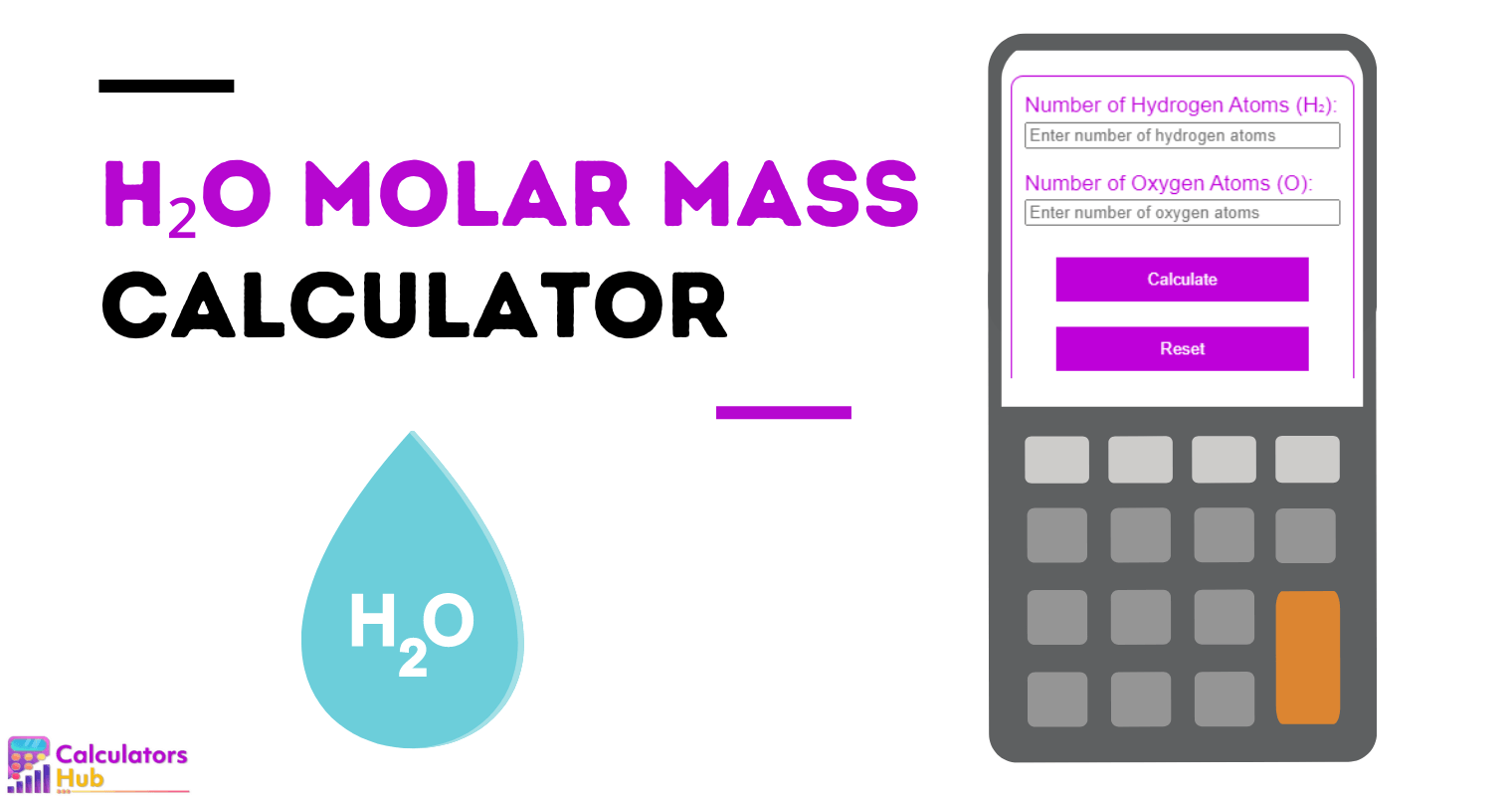
Molar Mass of H₂O: The Cornerstone of Chemistry and Life Itself
Molar Mass of H₂O: The Cornerstone of Chemistry and Life Itself
With a molar mass of precisely 18.01528 grams per mole, the molar mass of water (H₂O) serves as a foundational benchmark in chemistry—bridging theory and real-world application across biology, engineering, and environmental science. More than a mere number, this value underpins calculations in pharmaceuticals, metabolic processes, climate models, and industrial manufacturing. Understanding how this seemingly simple sum reflects water’s molecular structure and behavior reveals why molecular mass matters at the most fundamental level.
At its core, the molar mass of H₂O arises from the atomic weights of hydrogen and oxygen: hydrogen contributes 1.008 g/mol and oxygen 16.00 g/mol, yielding 2(1.008) + 16.00 = 18.016 when accounting for standard isotope averages. This modest mass belies water’s outsized influence—each molecule participates in vast networks of hydrogen bonding, solvent interactions, and dynamic equilibrium. As chemist and author Joseph Kirchhoff notes, “Water’s mass is minimal, but its role is monumental—its molar mass unlocks the key to countless molecular transformations.”
Calculating Molar Mass: The Formula and Its Precision
To determine molar mass, scientists sum the weighted atomic masses of all constituent atoms according to the molecular formula.For H₂O, this requires precise attention: two hydrogen atoms each carrying approximately 1.008 atomic mass units (amu), and one oxygen atom at roughly 16.00 amu. The formula is often expressed as H₂O—where whole numbers directly translate to gram-scale mass via the International Table of Constants, which defines the amu in terms of carbon-12 and oxygen-16 isotopes.
- Hydrogen: 1.008 g/mol × 2 = 2.016 g/mol
- Oxygen: 16.00 g/mol
- Total: 2.016 + 16.00 = 18.016 g/mol
The International Union of Pure and Applied Chemistry (IUPAC) recognizes 18.01528 g/mol as the standard, reflecting advancements in precision mass spectrometry and isotope ratio analysis.
Water: The Universal Solvent Driven by Molecular Mass
The molar mass of H₂O directly influences water’s role as the most effective solvent in biological and environmental systems. It facilitates hydrogen bonding—where oxygen’s partial negative charge attracts hydrogen from neighboring molecules—creating a dynamic network that dissolves ions, sugars, proteins, and gases.Without this precise mass, the energy gradients in cells, nutrient transport in rivers, and cloud formation in the atmosphere would collapse. “Imagine chemistry without water’s balanced mass,” explains hydrologist Dr. Elena Ríos.
“Its 18 g/mol allows that perfect balance—light enough to evaporate, dense enough to support life, and cohesive enough to sustain structure in living systems.” This dual quality enables water to act not only as a carrier but also as a participant in reactions—from enzyme catalysis to photosynthesis.
Industrial and Scientific Applications Depend on Accurate Molar Data
In laboratories and manufacturing, the molar mass of H₂O is non-negotiable. In pharmaceuticals, it dictates drug formulation, stability, and dosing—ensuring that every molecule delivers consistent therapeutic effect.Water’s mass also determines density anomalies: pure water reaches maximum density at 4°C, a trait critical to aquatic ecosystems where ice floats, insulating life beneath frozen surfaces. Metabolic Engineering and Energy Storage Biotechnological advances hinge on precise molecular accounting. In fuel cells using water decomposition (electrolysis), molar mass calculations determine hydrogen yield per mole of water—guiding efficiency and scalability.
Similarly, in particle accelerators and cryogenic systems, controlled phases of water—governed by its molar mass—enable exact manipulation of matter at microscopic scales.
Environmental Science and Global Climate: Water’s Hidden Heft
Climate models depend on water’s thermodynamic properties, rooted in its molecular mass. The high heat capacity (4.184 J/g°C) stems partly from hydrogen bonding, directly tied to molecular weight.This allows oceans to absorb and store vast amounts of solar energy, moderating Earth’s temperature, and driving weather patterns. Remote sensing and satellite data rely on accurate H₂O molar mass metrics to track evaporation, precipitation cycles, and glacial melt. “Water’s mass is a quiet orchestrator of planetary systems,” says climatologist Dr.
Marcus Wu. “Its 18 g/mol underpins the energy dynamics that sustain every ecosystem.” Without precise molecular mass data, forecasting droughts, floods, and climate shifts would become far less reliable.
The Precision of Modern Measurement and Its Implications
Advances in laser absorption spectroscopy, atomic mass spectrometry, and quantum chemistry refinement have elevated water’s molar mass determination to extraordinary accuracy.These technologies resolve isotopic distributions—such as deuterium and oxygen-18—enabling trace analysis critical to geochemistry, forensic science, and space exploration. For NASA and planetary missions, isotopically enriched water samples provide clues about past water activity on Mars. The same purity in measurement safeguards drug development, cross-continental chemical reactions, and industrial quality control.
The number 18.01528 isn’t just a label—it reflects a consensus forged by rigorous science.
In Short: The Molecular Key to a Universe of Function
The molar mass of H₂O, precisely 18.01528 g/mol, is far more than a static chemical figure. It represents the molecular foundation of life’s solvent properties, metabolic stability, climate regulation, and industrial utility.From cellular pathways to global weather, water’s mass governs dynamics that sustain ecosystems and technologies alike. As research continues to probe hydrogen bonding, quantum effects, and molecular interactions, this value remains a bedrock—testing, calibrating, and illuminating the intricate dance of matter and energy. Understanding water’s molar mass isn’t just about chemistry—it’s about grasping the very pulse of physical science.


Related Post

Sierra Lisabeth: The Entertainer of the Year in Fierce Demand
Panama City Beach Airport: Gateway to the Emerald Coast's Future
Listcrawler Br.: The Deckbiefer of Digital Discovery – How AI-Powered Platforms Revolutionize Market Research & Competitive Intelligence