Sculpting Life: How Mo Diagrams Reveal the Hidden Dance of O₂ in Molecular Motion
Sculpting Life: How Mo Diagrams Reveal the Hidden Dance of O₂ in Molecular Motion
Along the invisible front lines of chemistry, molecular behavior dictates the pulse of living systems—now crystallized in striking clarity through Mo Diagrams for O₂. These powerful visual tools transform abstract quantum states into tangible insights, revealing how oxygen molecules vibrate, rotate, and interact at the subatomic level. Mo Diagram For O₂ has emerged as an indispensable resource for researchers, educators, and industry scientists seeking to visualize and understand oxygen’s dynamic behavior beyond static models.
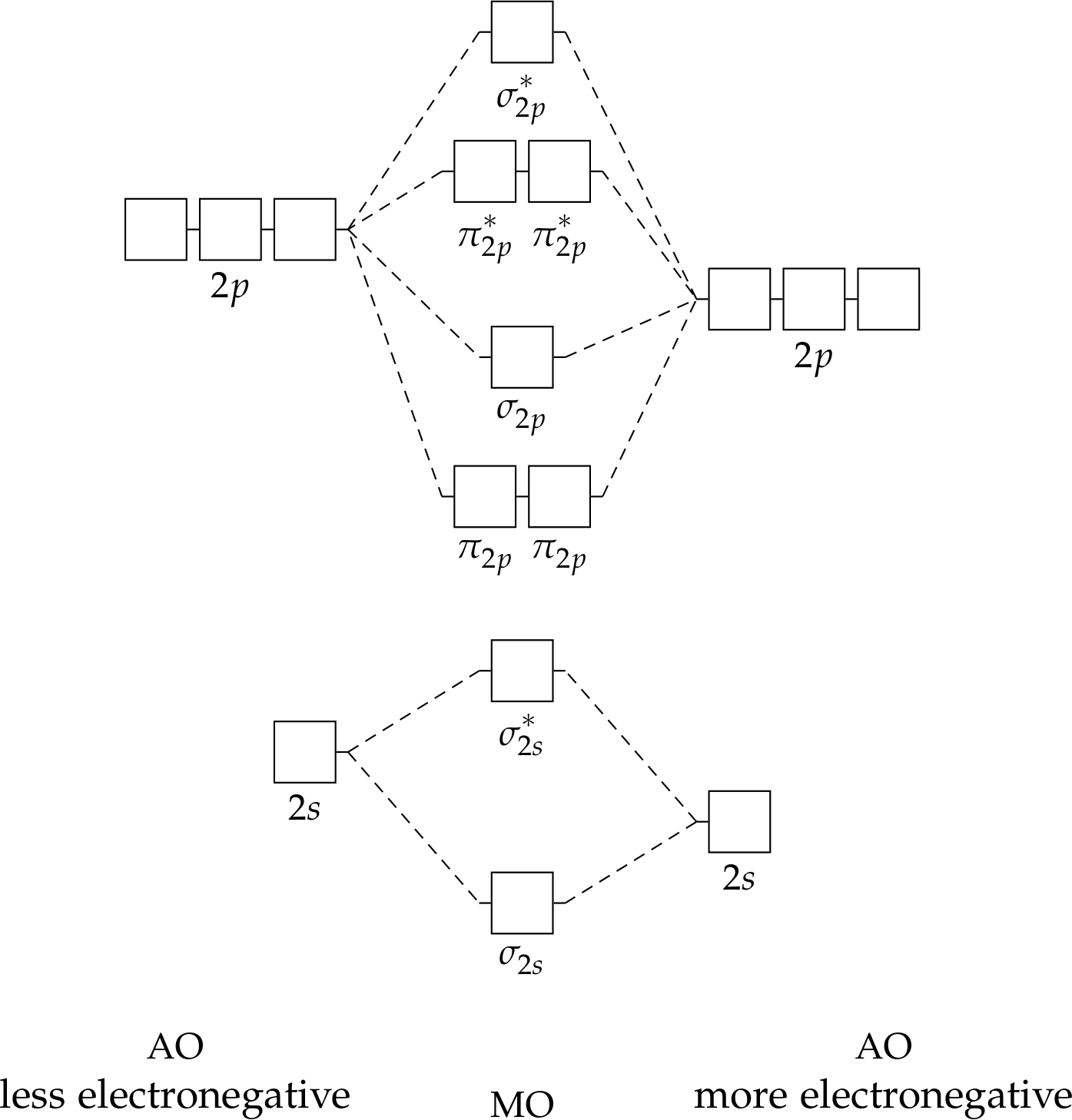
Mo Diagrams for O₂ map the energy levels and quantum states of the diatomic molecule O₂, demonstrating how molecular orbitals fill under real-world conditions. Picture, for instance, how electrons occupy bonding and antibonding orbitals such as σ2p and π*2p—states that determine whether O₂ remains diamagnetic or develops unique reactivity traits. Unlike simplified diagrams, these advanced representations incorporate experimental data and computational predictions to reflect actual energy distributions, bond strengths, and electronic configurations across temperature and pressure ranges.
At the core of Mo Diagram For O₂ lies a precise depiction of molecular orbital energetics. Oxygen’s paramagnetic nature—a key trait overlooked in basic chemistry teaching—finds intuitive explanation through relative orbital occupancies. While the 1σ and 2σ bonding orbitals are fully occupied, the 2σ antibonding orbital remains empty, and the π2p bonding orbitals host paired electrons.
This configuration directly explains O₂’s magnetic moment and low bond order of 2, foundational to its chemical stability and biological role.
Why Mo Diagrams Matter for Science and Industry Mo Diagrams For O₂ are not merely visual aids—they are analytical instruments that bridge theory and practice. In materials science, understanding O₂’s electronic transitions enables better design of semiconductor coatings and corrosion-resistant alloys, where oxygen diffusion impacts performance. In energy research, these diagrams clarify how O₂’s redox behavior underpins fuel cells and lithium-oxygen batteries, where oxygen reduction reactions (ORR) determine efficiency.Dr. Elena Markova, a molecular physicist at Imperial College London, notes: “O₂’s orbital structure, as illuminated by Mo Diagrams, reveals why it’s both essential and challenging to manipulate—surface catalysis depends on precise energy matching, and only accurate orbital visualization can guide optimizations.”
Each node and energy contour in the Mo Diagram For O₂ symbolizes fundamental quantum mechanical principles that govern molecular stability and reactivity. For example, the diagram visually captures the π-bonding delocalization critical to O₂’s inert pair effect and its weak bond reconstruction under oxidative stress.
These details matter: in medical diagnostics, where hypoxia and oxidative damage shape disease outcomes, molecular orbital maps help model how hemoglobin interacts with O₂ at the electronic level.
- Orbital Fill States: σ2p (fully filled), π2p (fully filled with paired electrons), 2σ (empty), and π*2p (empty, explaining paramagnetism).
- Energy Landscapes: Bonding orbitals delocalize electron density across atoms, stabilizing the molecule, while antibonding orbitals limit bond strength when partially occupied.
- Experimental Validation: Spectroscopic data from UV-Vis and electron paramagnetic resonance (EPR) consistently match the energy levels portrayed in Mo Diagrams For O₂.
- Predictive Power: Computational chemists use these diagrams to simulate reaction pathways, enabling breakthroughs in catalytic converters and targeted drug delivery systems involving reactive oxygen species.
Educators, too, have embraced Mo Diagrams as transformative tools.
The diagrams decompose complex orbital concepts into accessible visual narratives, empowering students to grasp why O₂’s triplet ground state drives radical biology, atmospheric chemistry, and combustion science. As Dr. Markova concludes: “In a world increasingly driven by molecular understanding, Mo Diagrams For O₂ don’t just depict O₂—they reveal the invisible choreography that sustains life and fuels innovation.”
The Mo Diagram For O₂ stands as a testament to how visualization elevates scientific insight.By rendering quantum behavior into clear, interactive models, it equips researchers and learners alike to navigate the molecule’s intricate dance—where every electron’s orbit tells a story of stability, reactivity, and life-sustaining function.



Related Post

Nude Female Models and the Evolving Dialogue Between Art, Identity, and Cultural Expression

