Unlocking Formaldehyde’s Secrets: The Essential Lewis Structure of CH₂O
Unlocking Formaldehyde’s Secrets: The Essential Lewis Structure of CH₂O
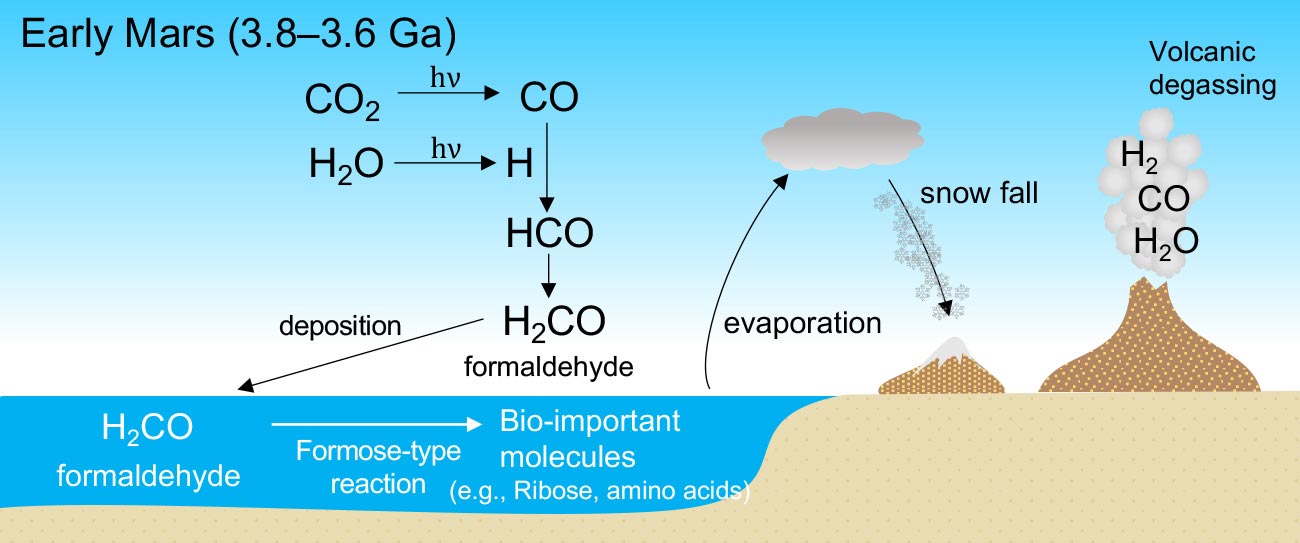
Formaldehyde (CH₂O) stands as a deceptively simple molecule with a profound impact across science, industry, and daily life. As the simplest aldehyde, its Lewis structure reveals more than just atomic arrangement—it exposes the foundation of its chemical behavior, reactivity, and utility. Understanding how carbon, oxygen, and hydrogen bond in CH₂O is critical for chemists, environmental scientists, and industrial researchers alike.
This article delves deep into the Lewis structure of CH₂O, decodes its formal charge distribution, explores resonance effects, and examines its real-world relevance—all anchored in precise chemical reasoning.
The Lewis structure for CH₂O begins with carbon at the center, bonded to two hydrogen atoms and one oxygen atom via single bonds, with a double bond forming between carbon and oxygen. This configuration satisfies the octet rule for all atoms and aligns with VSEPR theory to produce a trigonal planar molecular geometry.
Carbon, with four valence electrons, forms four bonds total—two as single bonds to hydrogen and one to oxygen, plus a critical double bond to oxygen. The oxygen atom holds six valence electrons, achieving stability through two single bonds and a lone pair. Together, the atoms share electrons in a way that balances energetics and molecular stability.
The Core Triple Bond Analogy (and Why It Doesn’t Exist) A common misconception is that CH₂O contains a C=O double bond as the sole key to its reactivity.In reality, resonance plays a pivotal role. Although the Lewis structure shows a fixed double bond between C and O, actual delocalization of π-electrons across the molecule generates resonance forms—transition structures that enhance stability without altering atomic positions. This shift between resonance contributors lowers the molecule’s energy and explains why CH₂O participates readily in chemical reactions.
“Resonance isn't just theoretical—it’s the invisible choreography enabling formaldehyde’s versatility,” notes Dr. Elena Torres, a physical chemist specializing in organic electrophiles. These dynamic electron distributions make CH₂O both a study in molecular symmetry and a reactive intermediate in green chemistry and environmental processes.
Resonance and Formal Charge: Refining the Lewis Picture While the standard Lewis structure for CH₂O shows carbon with four bonds and oxygen with two single bonds plus a lone pair, a closer look reveals formal charge calculations. Carbon carries zero formal charge—having four bonding electrons (two from each bond) and no lone pairs. Oxygen, with six valence electrons and a double bond (counted as four shared electrons), bears a formal charge of zero: 6 – [(4 + 2)/2] = 0.
The hydrogen atoms, with one bond each, also register as neutral. Yet these numbers mask the molecule’s subtle polarity. The electronegative oxygen pulls electron density toward itself, creating a partial negative charge (δ⁻) on oxygen and a partial positive charge (δ⁺) on the hydrogen-bearing carbon.
This charge distribution drives CH₂O’s strong dipole moment and enables its role as a proton donor in acid-catalyzed reactions.
Reactivity Hooks: How Structure Drives Function The precise geometry and bond hybridization in CH₂O underpin its diverse reactivity. As a planar aldehyde, its carbonyl group is sterically accessible, facilitating electrophilic addition—key in reactions forming hemiacetals, acetals, or organic salts. The double bond also allows nucleophilic attack, essential in formaldehyde’s role as a precursor in polymer synthesis and pharmaceutical intermediates.
Environmental concern adds urgency: CH₂O’s volatility and potential carcinogenicity prompt rigorous monitoring, particularly in indoor air and industrial emissions. “Understanding the Lewis structure isn’t just academic—it directly informs safety protocols and green chemistry initiatives,” observes Dr. Marcus Lin, an environmental chemist.
“Describing the electron stew keeps us ahead in risk assessment and sustainable application.”
Beyond Basic Structure: Applications Rooted in Electron Behavior The chemical story of CH₂O extends far beyond static bonding. Its resonance-stabilized form shapes reactions in atmospheric chemistry—contributing to formaldehyde emissions from paints, solvents, and vehicle exhaust. Inside the body, trace levels of formaldehyde influence metabolic pathways and trigger detoxification responses.
Industrially, controlled redox chemistry involving CH₂O serves organic synthesis, pharmaceuticals, and even disinfectant formulations. “Each bond, each charge, each resonance form is a clue,” says Dr. Lin.
“Deciphering this small molecule unlocks broader truths about molecular design and reactivity across scales—from labs to ecosystems.”
The Role of Visual Representation in Chemical Comprehension Though structured text dominates, visual clarity remains vital. Unlike ambiguous diagrams, a properly rendered Lewis structure for CH₂O—typically showing C=O and C–H (two single bonds), with an oxygen center holding a double bond, lone pairs, and consistent formal charges—provides an immediate snapshot of electron economy. General chemistry educators increasingly rely on such visual scaffolds to bridge theory and application, helping students grasp abstraction through reliable notation.
“A clean diagram isn’t decoration—it’s a communication tool that transforms complexity into clarity,” states Dr. Torres. In research labs, digital molecular visualization tools build on fundamental Lewis concepts to simulate reaction dynamics and predict behavior—extending the molecule’s impact beyond static representation into predictive science.
In sum, the Lewis structure of CH₂O is far more than a classroom illustration—it’s a vital framework for interpreting a molecule central to chemical innovation and environmental challenge.
Its double-bond resonance, formal charge neutrality, and electron distribution patterns illuminate reactivity, stability, and function. From air quality monitoring to organic synthesis, CH₂O’s structure guides discovery and safety. As chemistry evolves toward sustainability and precision, understanding such foundational models remains indispensable.
Formaldehyde’s story, written in electrons, continues to shape science and society—one bond at a time.


Related Post

Better Together: How Companionship Transforms Lives
Days Since July 1st: Time Is Ticking — Do This Before It’s Too Late
Logan Paul Takes a Jab at One of The Rocks Iconic Promos
Emma Watson’s Age and Journey: A Defining Chronicle from Child Star to Global Empowerment Icon

