Unlocking Neutralization: How Bronsted-Lowry Theory Clarifies Acid-Base Reactions
Unlocking Neutralization: How Bronsted-Lowry Theory Clarifies Acid-Base Reactions
When an acid and a base merge to form salt and water, a chemical dance unfolds—one governed by precise principles. From a Bronsted-Lowry perspective, this transformation is a textbook example of proton transfer: an acid donates a proton (H⁺), while a base accepts it. This foundation explains not only the simplicity of neutralization but also the intricate interplay of molecular identities behind everyday phenomena from antacids soothing stomach acid to industrial wastewater treatment.
With Bronsted-Lowry theory as our lens, the mechanics of neutralization emerge clear, revealing a reaction that is as predictable as it is vital.
The Bronsted-Lowry Framework: Protons, Acids, and Bases Redefined
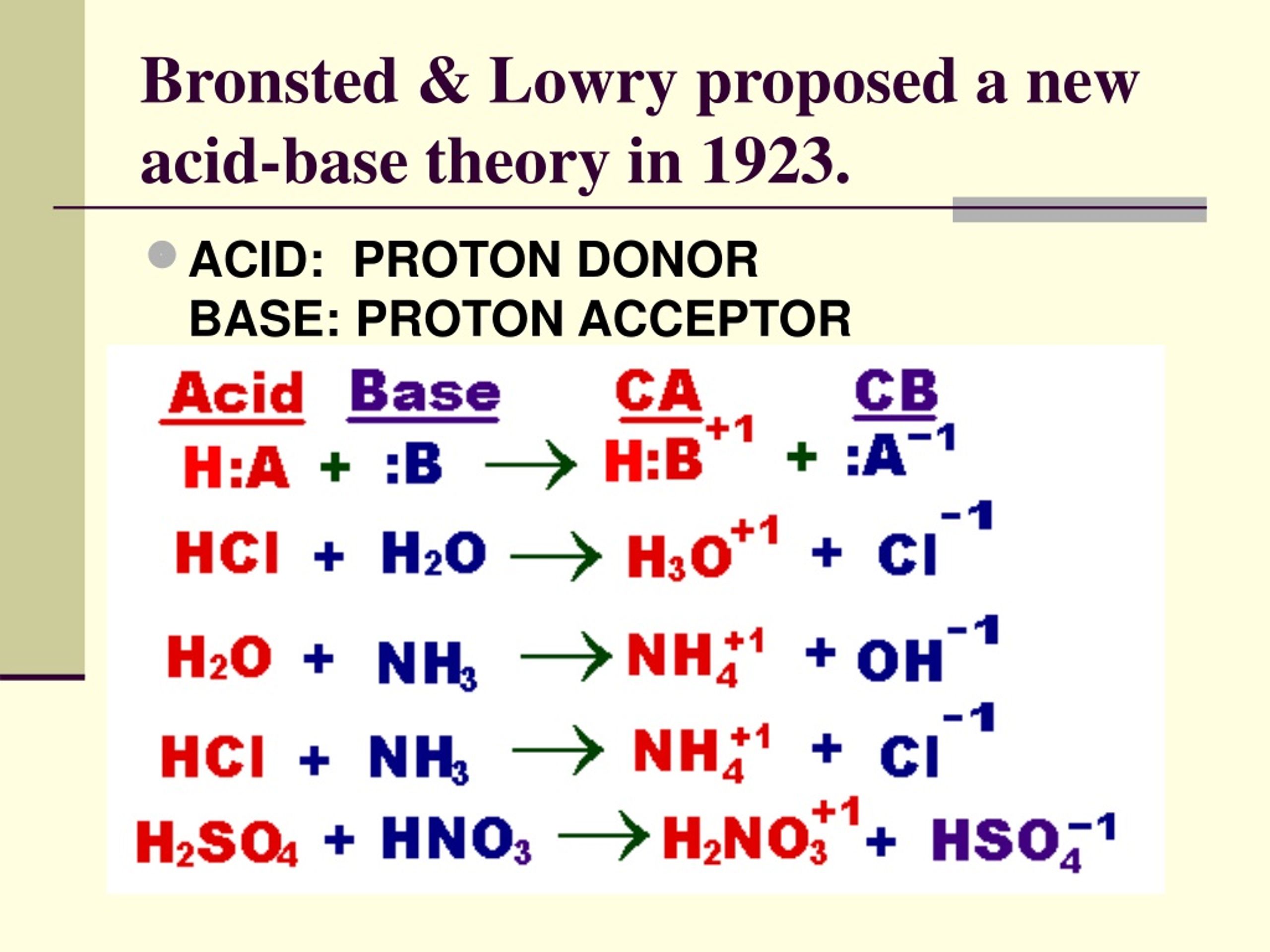
The Bronsted-Lowry definition refines classical acid-base theory by shifting focus from hydroxide ions (OH⁻) to proton exchange. According to this model: - An **acid** is any substance that donates a proton (H⁺), - A **base** is any substance that accepts a proton.This definition transcends water-based systems, enabling accurate analysis of reactions in non-aqueous solvents and complex biological environments. In neutralization, the acid’s proton becomes the key player, transforming into a stable conjugate base while its conjugate acid forms. Meanwhile, the base’s hydroxide ion accepts this proton to generate water and a new conjugate acid.
This proton-sharing ritual ensures charge balance and explains why the hallmark products are always water and a neutral salt.
Mapping Neutralization: From Bronsted Conjugate Pairs to Salt Formation
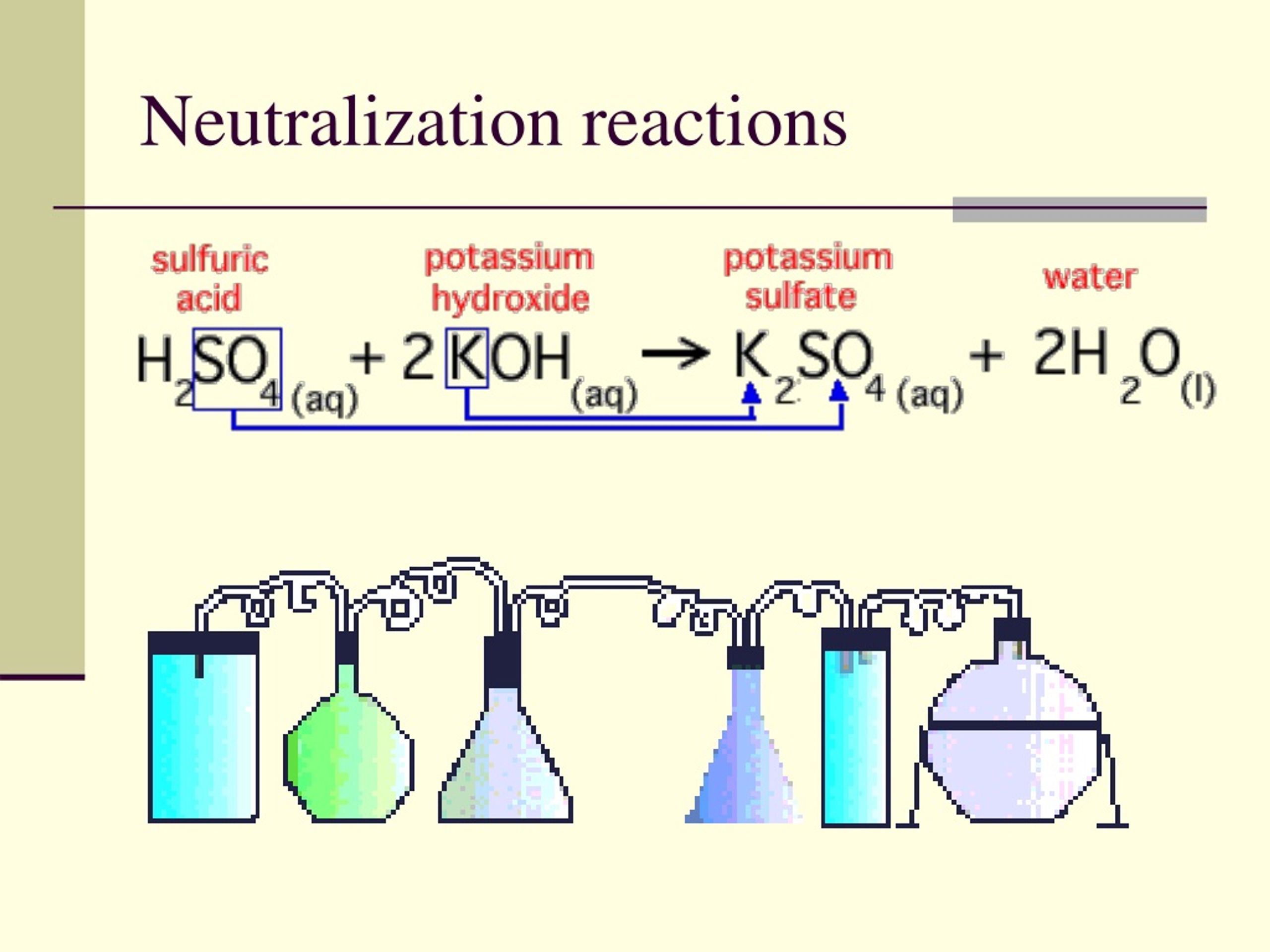
Consider a canonical neutralization reaction: hydrochloric acid (HCl) reacting with sodium hydroxide (NaOH). Using Bronsted-Lowry theory: - HCl acts as the acid, donating H⁺ to become Cl⁻, its conjugate base.- OH⁻ serves as the base, accepting H⁺ to form H₂O, its conjugate acid. The proton transfer yields: $$ \text{HCl} + \text{NaOH} \rightarrow \text{NaCl} + \text{H}_2\text{O} $$ This equation encapsulates the proton economy: H⁺ moves from acid to base, reconstructing molecular identities. The sodium chloride (NaCl) forms as a neutral salt, demonstrating how acid-base synergy delivers stability from opposites.
Each step preserves charge and mass, reinforcing the theory’s predictive power in both lab and nature.
Proton Transfer in Action: Why Neutralization Matters Beyond the Lab
Neutralization is far more than a textbook example—it drives practical applications grounded in Bronsted-Lowry principles. - In medicine, antacids like magnesium hydroxide bridge excess H⁺ from stomach acid (HCl, H₂SO₄), restoring gastric pH.- Environmental systems use neutralization to treat industrial effluent, reducing acidity or alkalinity to safe discharge levels. - In agriculture, soil amendment with lime (CaO) neutralizes acidic soils, enhancing nutrient availability. These uses thrive because Bronsted-Lowry theory clarifies how proton exchange regulates reactivity.
It reveals why certain bases counteract specific acids and ensures reactions proceed efficiently toward equilibrium. Without this molecular insight, optimizing neutralization for safety and efficacy would rely on guesswork.
The Balancing Act: Equilibria, Specificity, and Selectivity in Neutralization
Not all acid-base pairs behave equally.Bronsted-Lowry theory illuminates why HCl fully neutralizes NaOH—strong acids and strong bases dissociate completely, driving the reaction nearly to completion. In contrast, weak acids like acetic acid (CH₃COOH) or bases like ammonia (NH₃) form partway, establishing dynamic equilibria where partial proton transfer yields temporary neutralization until equilibrium stabilizes. This selectivity shapes reaction design: - Selective neutralization enables precise pH control in biochemical processes, preventing over-acidification or alkalization.
- Buffer systems exploit weak acid-conjugate base pairs to resist pH shifts—another application deeply rooted in proton transfer logic. Thus, Bronsted-Lowry theory not only explains neutralization but also guides its strategic deployment in science and industry.
In every case, the transfer of a proton defines the reaction’s essence—a molecular tug-of-war with clear rules.
By applying Bronsted-Lowry theory, neutralization ceases to be a mere chemical equation and becomes a powerful, predictable force shaping health, environment, and technology. Nature’s elegance lies in such precise proton exchanges, turning elemental simplicity into functional complexity.
Understanding neutralization through Bronsted-Lowry principles transforms abstract chemistry into actionable knowledge—proving that the heart of chemical transformation beats steadily beneath everyday reactions.


Related Post
Decoding the Chaos: The Limits and Surprises of Google Translate 100 Times